Introduction: A Unifying Target in ALS Pathogenesis
In nearly 97% of ALS cases, the DNA/RNA-binding protein TDP-43 mislocalizes from the nucleus to the cytoplasm, forming insoluble aggregates and disrupting critical cellular functions. This pathological hallmark links both sporadic and familial ALS, placing TDP-43 at the center of ALS pathogenesis and making it a compelling target for both therapeutic development and biomarker discovery.
Despite this, the field has faced substantial barriers: the dual toxic effects of nuclear loss-of-function and cytoplasmic gain-of-function, patient-to-patient variability, and a lack of reliable tools for early detection and therapeutic monitoring.
At Target ALS, 30% of our funded research portfolio is now focused on advancing TDP-43 science. This investment spans basic molecular studies to antisense therapeutics, small molecule discovery, gene therapy, and biomarker development—all underpinned by our shared core resources and collaborative research infrastructure.
Scientific Challenge: A Protein with Multifaceted Toxicity
TDP-43’s normal function involves tightly regulated RNA splicing, transport, and stabilization. When mislocalized, it forms hyperphosphorylated aggregates, contributing to a cascade of dysfunctions including transcriptome-wide misregulation, axonal degeneration, and mitochondrial impairment.
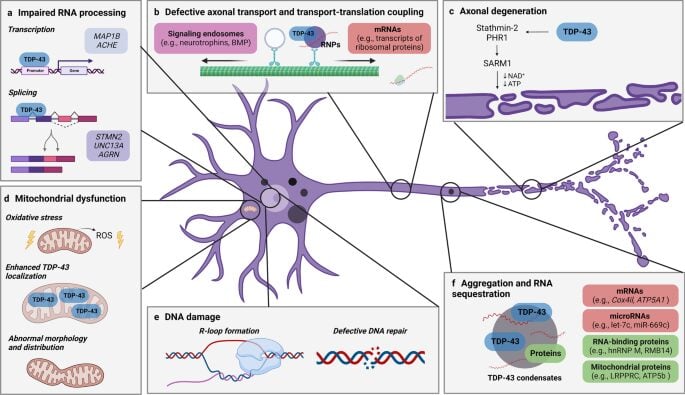
(a) impaired RNA processing, (b) defective axonal transport and translation, (c) axonal degeneration, (d) DNA damage, (e) mitochondrial dysfunction, and (f) aggregation with RNA sequestration. Each pathway highlights how abnormal TDP-43 localization and function disrupts neuronal health, contributing to motor neuron degeneration characteristic of ALS. (Lépine et al, 2022)
Therapeutic strategies must walk a tightrope—clearing or suppressing pathological TDP-43 species without compromising its vital nuclear role. Further complicating matters, the mechanistic contributors to its aggregation—such as post-translational modifications, stress granule dynamics, and RNA-binding dysregulation—are complex and not yet fully delineated.
TDP-43 Research Hurdles:
- TDP-43 causes damage in two ways: by losing its normal role in RNA processing in the cell nucleus and by forming toxic clumps in the cytoplasm. This means treatments need to both clear harmful clumps and restore healthy function.
- Studies of ALS and FTD patients show that there are different types of TDP-43 aggregates, which may each need tailored tests and treatments.
- Therapies must target only the harmful version of the protein without affecting the healthy form.
- Because it’s unclear whether treatment can help once the damage is advanced, early detection through reliable biomarkers is critical.
Target ALS’s Coordinated Approach
Given the key role of TDP-43 in ALS, Target ALS has put significant funds behind TDP-43 research in the past, and continues to do so with 30% of the Target ALS research portfolio dedicated to this area of research. Our strategic approach is to advance our understanding of TDP-43 dysfunction and toxicity by funding research to study the details of TDP-43 pathology and to advance development of new therapeutics and biomarkers targeting TDP-43 through funding opportunities and by providing hard-to-get tools and resources to enable the research. . Progress in one of these areas cannot be made without progress in the other. For example, TDP-43-directed therapeutics must be accompanied by biomarkers of TDP-43 pathology to enable successful clinical trials.
Our strategy addresses this complexity through targeted funding, risk-tolerant support for early innovation, and open-access resources that enable reproducibility and cross-lab validation.
Key pillars of our approach:
- Therapeutic discovery – Funding diverse, mechanistically grounded therapeutic strategies to modulate TDP-43 pathology
- Biomarker development – Funding to build tools for non-invasive detection and patient stratification
- Research-enabling infrastructure – Our Core Resources (link here) Offer access to postmortem tissue, longitudinal biofluids, validated reagents, preclinical models, and support
Therapeutic Strategies in Focus:
At least 70 different drugs have been tested or are being tested in on-going clinical trials for ALS. Many of these treatments aim to dampen disease factors that arise after motor neuron degeneration has been triggered. Target ALS-funded projects are taking bold, mechanistically driven approaches to tackle TDP-43 pathology at its roots. These efforts focus on novel strategies—ranging from disrupting toxic post-translational modifications and pathogenic protein–protein interactions, to using machine learning and computational modeling to design therapeutics highly specific to toxic forms of TDP-43. Together, these projects represent a new wave of TDP-43–directed therapies that aim to intervene at molecular choke points early in the ALS disease cascade and may even be used to prevent onset of clinical symptoms. Below are examples of Target ALS-funded projects and partnerships working to advance TDP-43-targeted therapeutics through diverse modalities.
RNA-Targeting: Antisense Oligonucleotides (ASOs):
With the success of RNA-based therapeutics for genetic forms of ALS – like the antisense oligonucleotide (ASO) Tofersen for SOD-1 ALS – multiple research groups have aimed to apply this therapeutic strategy to TDP-43 and downstream pathology. Target ALS supports several groups using ASOs to directly target TDP-43 or to target dysregulated RNAs downstream of TDP-43 dysfunction:
- n-Lorem: Advancing personalized ASO therapies to reduce toxic cytoplasmic accumulation while retaining normal function of wild-type TDP43 in the nucleus.
- Northwestern and NuCyRNA: Developing a dual-targeting oligonucleotide technology that can knock down modifier genes to mitigate TDP-43 pathology and correct specific TDP-43 loss-of-function phenotypes.
Targeting Cytoplasmic Aggregation and Stress Granules:
Stress granules are membraneless organelles that form in cells in response to cellular stressors, like heat shock or DNA damage. The Integrated Stress Response (ISR) is a pathway that can also be activated in response to these stressors. In ALS, stress granules and ISR activation occur in motor neurons and other cell types of affected individuals. Stress granules are even implicated in pathology and interplay with TDP-43 cytoplasmic aggregation. Target ALS supports and has supported several groups looking to impact ALS progression by developing therapeutics that target stress granule formation or ISR activation in disease.
- Dewpoint Therapeutics: Small molecules disrupting TDP-43’s recruitment into stress granules—a key early event in aggregation.
- Denali / UCSD: Targeting OTUD4, a stress granule component, to prevent TDP-43 inclusion body formation.
- eIF2B activators – Denali: Preclinical work on eIF2B activators targeting the Integrated Stress Response in ALS, but recent Phase II trial failures highlight the challenge of treating this complex disease and underscore the need for deeper biomarker insights to interpret outcomes.
Protein-targeting Strategies:
Many groups designing therapeutics for ALS seek to target dysfunctional TDP-43 protein directly. Whether they are altering how TDP-43 binds with protein partners or its modification by kinases, the following candidate therapeutics supported by Target ALS all aim to disrupt toxic TDP-43 aggregates at the protein level.
- Acelot: Using in silico simulations and proprietary experimental assays to develop small molecules that directly target aggregated TDP-43 and restore downstream function.
- Neumora Therapeutics: Inhibition of CK1δ, blocking pathological TDP-43 phosphorylation at Ser409/410.
- McGurk Lab: Targeting Tankyrase–TDP-43 interactions to mitigate stress-induced aggregation.
- Latouche-Buratti-Cintrat-Sperandio Consortium: Using structural modeling of TDP-43 to identify small molecules that detect or block its aggregation, applying a rational, structure-guided approach to ALS therapy.
- Mabylon: AAV-based delivery of intrabodies to intracellularly bind and neutralize aggregated TDP-43—a novel modality that prevents seeding and aggregation of pathological TDP43 and leaves the normal form of TDP43 intact.
Strategies to restore RNA processing downstream from TDP-43 dysfunction:
While many therapeutic efforts target the toxic gain-of-function (GOF) effects of cytoplasmic TDP-43, a growing number of innovative strategies are focused on correcting the loss of its essential nuclear functions. TDP-43 plays a critical role in regulating RNA splicing and transcript stability; its nuclear depletion leads to widespread dysregulation of downstream targets. Target ALS is supporting several projects that aim to correct the downstream consequences of TDP-43 nuclear loss.
Cryptic Exon-Directed Therapies
- Trace: Developing a splice-switching ASO to correct UNC13A misregulation—a key downstream effect of TDP-43 loss—with the goal of restoring synaptic function and rescuing motor neuron health in ALS.
- QurAlis: Developing ASOs to restore TDP-43-dependent splicing of key genes like STMN2, with Target ALS supporting their broader pipeline through funding and biomarker resources.
Learn more about these projects in detail here.
Biomarkers: Pioneering Non-Invasive Tools for TDP-43 Detection
A major bottleneck in ALS research has been the absence of fluid-based biomarkers that reflect central pathology.
With support from grants by Target ALS and the ALS Association, Dr. Robert Bowser’s team at the Barrow Neurological Institute (BNI) has developed a highly sensitive immunoassay using the MSD platform to detect full-length TDP-43 in plasma and serum—an important step toward fluid-based diagnostics for ALS. The assay detects decreased total TDP43 in blood from ALS patients compared to healthy control. Dr. Bowser and team hypothesize that a reduction in total TDP43 may be telling us that there is a related increase in truncated, pathological forms of TDP43 in these patients.
In parallel, the BNI-AC Immune Consortium, funded in 2022, developed assays to measure TDP-43 in platelets, identifying similar reductions in TDP43 in patient samples. Antibodies may be found via the Target ALS Reagents Core or via the investigators and methods for the assay have been published (ref). This advancement holds promise for improving diagnosis and monitoring treatment response by utilizing blood-based biomarkers.
Read more about these two projects here.
Next-Gen Diagnostics: Novel Approaches to Detecting and Understanding TDP-43 Pathology
As our understanding of TDP-43 in ALS deepens, researchers are pioneering new tools to detect its toxic forms earlier, more accurately, and less invasively. From seed amplification assays and nasal swabs to cryptic exon biomarkers in biofluids, these cutting-edge approaches aim to transform how we diagnose, monitor, and ultimately treat TDP-43-driven disease.
Seed amplification assays:
Seed amplification assays (SAAs) like RT-QuIC were developed to diagnose prion diseases by specifically triggering the aggregation of prion proteins in the biofluids or tissue of affected individuals. Given that TDP-43 aggregation is a hallmark of ALS, researchers are looking to apply this cutting edge technology to ALS diagnosis. SAAs have the potential to diagnose ALS or track its progression from less invasive biofluids like serum or plasma. Target ALS is supporting a number of projects advancing the development of this diagnostic technology.
- Dr. Sarah Smith of Harvard/MGH is pioneering a novel “digital seed amplification assay” to get a binary yes/no answer on whether patient biofluids have TDP-43 aggregates.
- Dr. Javier Oroz of the Ramón y Cajal Institute, Spain is developing multiple methods of measuring TDP43 that will ultimately allow for an understanding of how TDP-43 aggregate species change during disease progression.
- The Polymenidou-Zanusso-Legname consortium developed a nasal swab-based RT-QuIC test, similar to a COVID-19 test, to detect small traces of TDP-43 aggregates in ALS and FTD patients. You can find the promising outcomes of their research here.
Cryptic exon biomarkers:
Capitalizing off of the knowledge of TDP-43’s critical role in regulating RNA splicing, multiple groups hope to utilize cryptic exons and peptides formed in the absence of nuclear TDP-43 to diagnose ALS or track its progression in patient biofluids.
- The Ward-Fratta-Petrucelli consortium has paved the way for TDP-43 dysfunction-based biomarkers, investigating the potential to use cryptic peptides found in patient blood or CSF as biomarkers of TDP-43 loss of function and are advancing assays with high sensitivity for the cryptic exon HDGFL2. Read more about their research here.
- Exciting technological advancement from Dr. Anja Schneider’s lab at DZNE has allowed for robust isolation of neuronal extracellular vesicles (EVs) in patient biofluids. Their consortium with scientists from Novartis, KU Leuven and Oxford University is taking advantage of these advancements, developing capabilities to detect both TDP-43 and cryptic exons found in EVs in blood. Success from this project could allow for less invasive diagnosis and disease monitoring.
- Several new consortia were funded in 2024 to deeply explore cryptic exons and peptides as ALS diagnostics.
Learn more about Target ALS supported Biomarker projects here.
Future Outlook on TDP43-related therapeutics
The focus on TDP43 therapeutics has begun to yield several mature programs poised to enter clinical trials over the next year. The significant effort on biomarkers fuels optimism that those clinical trials will have key biomarker assays to utilize in their clinical trials. The field awaits clinical data from many of these new, promising therapies which will be highly informative with respect to refining the central dogma and future drug discovery efforts.
Despite this momentum, several challenges to targeting TDP-43 remain:
- Preserving physiological function: Therapeutics must distinguish pathogenic from functional TDP-43 species.
- Disease heterogeneity: ALS subtypes related to different forms of TDP43 aggregates or co-pathologies (e.g., other protein aggregates, neuroinflammation, mitochondrial dysfunction) may require disease stratification biomarkers and combinatorial therapy
- Translation to clinic: the field relies on a combination of imperfect models to test and predict therapeutic activity leaving the potential for a gap in translation from bench to clinic
We welcome new collaborators and partners committed to tackling ALS at its molecular core. Target ALS is committed to providing tools and resources (https://www.targetals.org/research/resources-for-scientists/) to accelerate research on TDP-43 and basic mechanisms underlying the disease.



