Multicenter Human Postmortem Tissue Core
The Postmortem Tissue Core integrates six geographically distributed academic ALS centers with expertise in ALS and postmortem tissue banking. It is led by co-directors, Drs. Robert Bowser and Brent Harris. For any questions or to inquire about new or existing tissue requests, please contact Marina Selenica via email at ms4739@georgetown.edu.
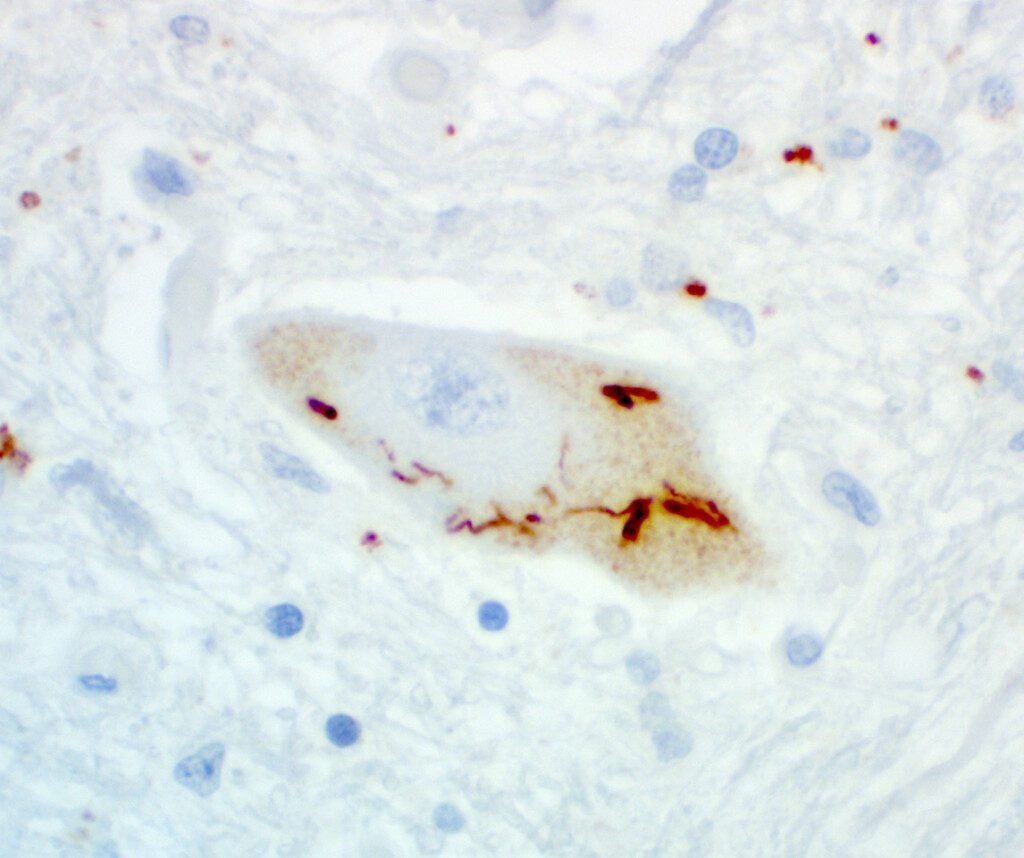
neuronal pTDP43 staining, antibody courtesy of Petrucelli Lab, Mayo Clinic. Brain image from Target ALS Post-mortem tissue bank, courtesy of Dr. Harris, Georgetown University
The core is administered by:
- Robert Bowser, Ph.D. (Barrow Neurological Institute)
- Brent Harris, M.D. Ph.D. (Georgetown University)
- Matthew Harms, M.D. and Neil Shneider, M.D. Ph.D. (Columbia University)
- John Ravits, M.D. Ph.D. (University of California, San Diego)
- Cindy Ly, M.D. Ph.D. (Washington University, St. Louis)
- Marina Selenica (Program Manager of PM core, Georgetown University)
- Colin Smith, MBChB (University of Edinburgh, Scotland)
The postmortem tissue collection consists of multiple brain and spinal cord regions from 292 sALS cases, 91 fALS, 39 FTD, and 110 non-neurological controls. We have defined standard operating procedures for tissue acquisition and dissection, processing, storage, histopathological characterization and QC analysis, all specifically optimized for ALS research. Tissue inventories from our multiple core sites and the corresponding de-identified clinical metadata are linked using platforms developed by the Center for Innovation & Bioinformatics. Genomic raw data files are available in the data portal.
To request samples, please click here to complete the form. Please note that we have a new application portal. Click here to learn more.
Researchers using samples and data from Resource Cores retain full ownership of their ideas and results, without authorship requirements or intellectual property concerns. The Target ALS PM Core Request Form includes detailed instructions for requesting samples from the Core. Researchers pay for shipping of samples with an additional transmittal fee for pharmaceutical and biotech companies.
Please direct any questions to Marina Selenica (ms4739@georgetown[dot]edu).
Process and Timeline For Obtaining Samples For Research
- Applications are reviewed on a rolling basis within two weeks of receipt, using established criteria that emphasize experimental feasibility and appropriateness of sample sizes and quantities.
- After the initial review, a direct phone call is scheduled with the Core Co-Directors Dr. Robert Bowser (robert.bowser@dignityhealth.org) and Dr. Brent Harris (bth@georgetown.edu) to address any questions or concerns, after which the best samples to suit your needs will be identified across our Core sites.
- If your assays/staining procedures have not been validated on human CNS tissues, we will arrange to send a blinded test sample set first to help with assay development.
- Once the samples have been identified, MTAs with the standardized language will be provided for each Core Site that will be sending tissue. Separate MTA language for academic/non-profit and industry/for-profit requests has been standardized and pre-approved by each Core Site. These MTAs can be provided upon request at any time. The local Site Coordinators will prepare the tissues for shipment so that they can be shipped as soon as the MTAs are executed.
- Researchers pay for shipping of samples. There is an additional transmittal fee requested for industry/for-profit requests of $50 per slide and $200 per frozen tissue sample.
Our goal is to say “yes” to every request; thus, the Core Co-Director works closely with each researcher to help optimize experimental plans, validate assays, and ensure that the researchers are always thinking about the next step — such as identifying potential biomarkers for eventual clinical trials.
For a post-doc, new investigator or established scientist with an entirely new idea, our goal is to rapidly provide the resources necessary to obtain at least preliminary results (“discovery”), and then foster further development and collaboration as the idea evolves. Often, we can facilitate collaborations with established labs already using our Cores or invoke other existing Core Resources to provide complementary data and results. New ideas can be tested rigorously within weeks, rather than the months-to-years that would normally be needed to apply for grants, get funded, establish lab assays locally, and conduct preliminary experiments.
New Target ALS Application Portal
We are in the process of changing our online grant application portal.
- You will need to create a new user account, called a Blackbaud ID, even if you have applied to our grants in the past.
- You will be able to share your applications with co-applicants (consortium members) or other key stakeholders in your organization.
- If you have a current Target ALS grant, you will not see it in this new application portal (we’re working on unifying them).
- Add noreply@yourcause.com to your safe senders list to make sure you receive our emails and notifications.

