| ALS 101 What Is RNA Splicing? Think of your DNA as a giant cookbook, and each gene is a recipe. To make something from that recipe, your body creates a copy of it called RNA. But that copy isn’t ready to use right away, it has to be edited first. That’s where splicing comes in. Splicing is like cutting out the unnecessary bits of a recipe so you’re left with only the steps and ingredients you need. The body trims out non-useful sections (called introns) and stitches together the useful ones (called exons) to make a final, working version of the instructions. What Are RNA Splicing Errors? RNA nucleotide sequences are edited through a “splicing” mechanism to create the genetic code unique to each protein. Sometimes, the body makes a mistake during the RNA editing process. This is called a splicing error. It’s like accidentally cutting out an important step in a recipe or leaving in a mistake. When this happens, the final instructions are wrong. As a result, your body might produce a faulty protein, or no protein at all. These proteins are critical to keeping cells healthy, so splicing errors can contribute to many diseases, including ALS, especially when they affect nerve cells. Antisense oligonucleotides (ASOs) are small, lab-made pieces of genetic material designed to bind to RNA in cells. By attaching to specific RNA sequences, ASOs can block or change how certain genes are used, either by reducing harmful proteins or helping the body make more of a helpful one. In ALS research, ASOs are being explored as a way to target the root causes of the disease at the genetic level. Key survival markers are biological signs that help predict how a disease like ALS will progress or how long a person might live with it. These markers can include specific proteins in the blood or spinal fluid, levels of gene activity, or changes in nerve cell health. In ALS research, improved survival markers often signal that a treatment is having a protective effect on the nervous system, even before major physical changes are seen. |
A multi-institutional team from Trace Neuroscience, Stanford, University of Michigan, and FMP Berlin is developing a promising and exciting new therapy that corrects RNA splicing errors in the UNC13A gene; a mistake seen in many ALS cases and linked to faster disease progression.
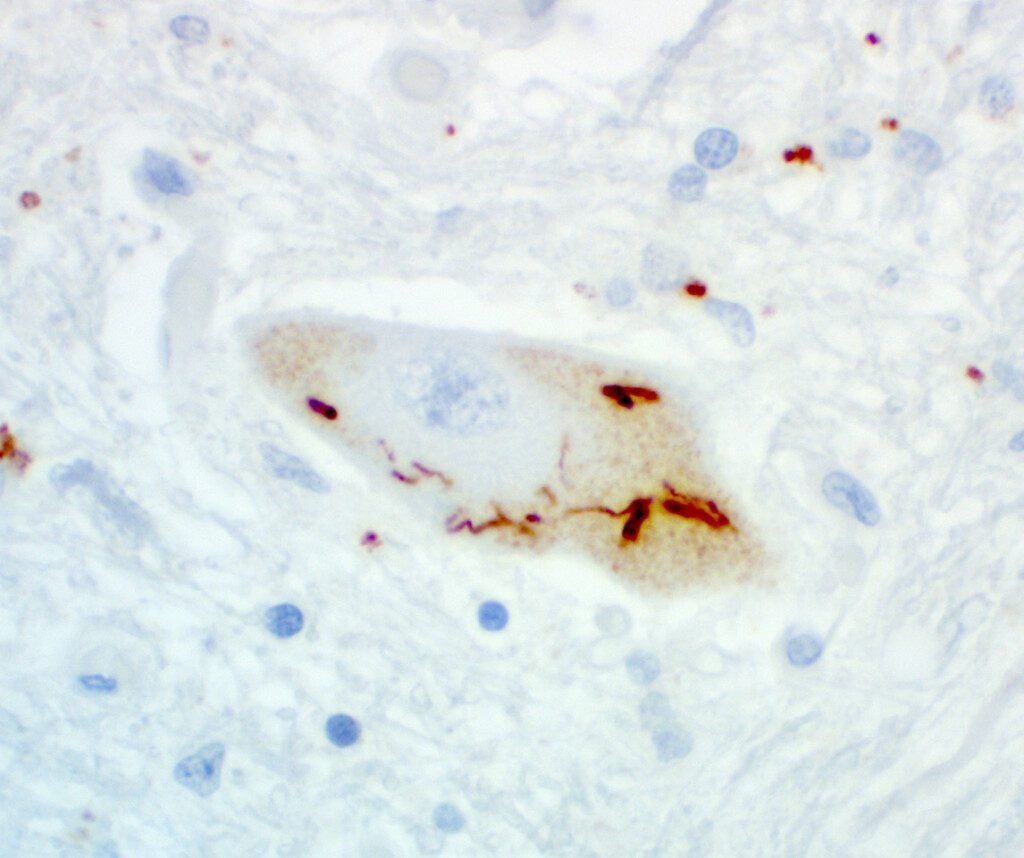
UNC13A is essential for synaptic communication (the way nerve cells transmit messages). In ALS, UNC13A mutations have been associated with increased risk of developing ALS and shorter survival times. In addition, the misplacement of a protein called TDP-43 causes UNC13A to be improperly spliced, leading to lack of proper protein formation and loss of its normal function. This mis-splicing is seen in many ALS cases and is linked to faster disease progression and worse survival outcomes.
This collaborative consortium has developed antisense oligonucleotides (ASOs), i.e. precision molecules that correct this faulty splicing. These ASOs have shown remarkable potency in preclinical models, restoring proper UNC13A expression in neurons derived from stem cells and in genetically engineered mice.
The results are compelling. When UNC13A is corrected in TDP-43-depleted neurons, researchers observed:
- Improved synaptic communication, with more frequent and stronger signal transmission between nerve cells.
- Restored neuronal network synchrony, a critical element of functional brain circuitry.
- Reduced dysfunction in key biomarkers and improved survival signals, even in models designed to mimic severe forms of ALS.
Beyond cellular models, the team has engineered a humanized mouse model that mimics the exact splicing error found in people with ALS. Using these mice, they found that even low doses of the ASO were sufficient to correct UNC13A splicing errors. They are now studying how UNC13A restoration affects motor function, circuit connectivity, and disease biomarkers. This lays the groundwork for future human trials.
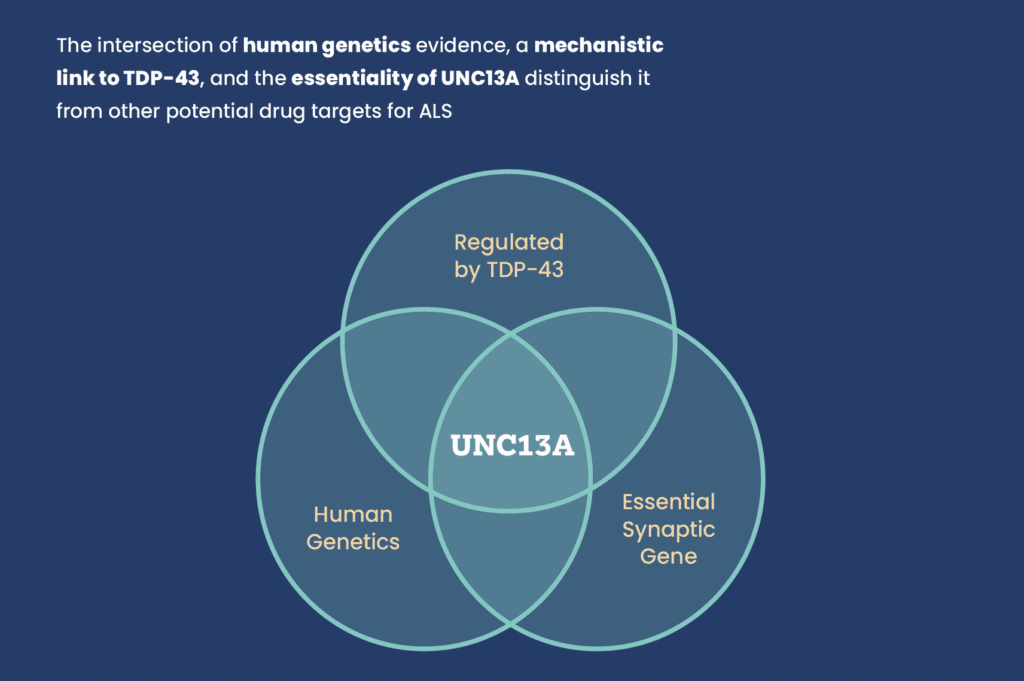
This research not only confirms UNC13A as a genetically validated, mechanistically sound target, but also represents a therapeutic strategy that could be effective regardless of genetic background. That makes it especially promising for the broader ALS population, many of whom do not have known gene mutations.
💡 Key Takeaway:
Correcting UNC13A mis-splicing could offer a broadly applicable therapy for most people with ALS. With early data showing restoration of neuron function and synaptic health, this work is a promising example of how understanding the genetic and mechanistic underpinnings of ALS can unlock transformative new treatments.